Brain tumors - primary
Highlights
Primary Brain Tumors
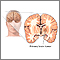
Primary brain tumors are tumors that start in the brain. There are many types and subtypes of primary brain tumors; some are benign, others malignant. Examples include gliomas, meningomas, medullablastomas, pituitary adenomas, and central nervous system lymphomas.
Causes
The exact causes of primary brain tumors are unknown. Genetic factors and inherited disorders play a role in some types of brain tumors.
Risk Factors
Risk factors for brain tumors vary according to the type of tumor. Some types of tumors are more prevalent in men than in women. Some types of brain tumors usually occur in children, while others are more common in older people.
Prognosis
Survival rates in people with brain tumors depend on many different variables:
- Type of tumor
- Location and size of tumor (these factors affect whether or not the tumor can be removed surgically)
- Tumor grade
- Patient's age
- Patient's ability to function
- How far the tumor has spread
Symptoms
Brain tumors produce a variety of symptoms including headache, seizure, and neurological changes. Symptoms may be subtle and gradually become worse or they may occur very rapidly.
Diagnosis
Diagnosis of brain tumor involves a neurological exam and various types of imaging tests. Imaging techniques include magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) scan. Biopsies may be performed as part of surgery to remove a tumor, or as a separate diagnostic procedure.
Treatment
The standard approach for treating brain tumors is to reduce the tumor as much as possible using surgery, radiation treatment, or chemotherapy. Such treatments are typically used in combination with each other.
New Drug Approval
In 2012, the Food and Drug Administration (FDA) approved the first drug specifically formulated for children with brain tumors. Everolimus (Afinitor Disperz) is a pediatric dosage drug used to treat the rare brain tumor called subependymal giant cell astrocytoma (SEGA).
Introduction
Brain tumors are composed of cells that exhibit abnormal growth in the brain.
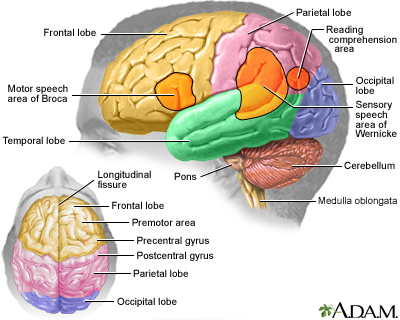
They can be benign (noncancerous, meaning that they do not spread elsewhere or invade surrounding tissue) or malignant (cancerous).
Cancerous brain tumors are further classified as either primary or secondary tumors.
Primary Brain Tumors
Primary tumors start in the brain, whereas secondary tumors spread to the brain from another site such as the breast or lung. (In this report, the term "brain tumor" will refer mainly to primary malignant tumors, unless otherwise specified.)
Benign Brain Tumors. Benign tumors represent half of all primary brain tumors. Their cells look relatively normal, grow slowly, and do not spread (metastasize) to other sites in the body or invade brain tissue. Benign tumors can still be serious and even life threatening if they are in vital areas in the brain where they exert pressure on sensitive nerve tissue or if they increase pressure within the brain. While some benign brain tumors may pose a health risk, including risk of disability and death, most are usually successfully treated with techniques such as surgery.
Malignant Brain Tumors. A primary malignant brain tumor is one that originates in the brain itself. Although primary brain tumors often transmit cancerous cells to other sites in the central nervous system (the brain or spine), they rarely spread to other parts of the body.
Brain tumors are generally named and classified according to either of the following:
- The type of brain cells from which they originate
- The location in which the cancer develops
The biologic diversity of these tumors, however, makes classification difficult.
Secondary (Metastatic) Malignant Brain Tumors
A secondary (metastatic) brain tumor occurs when cancer cells spread to the brain from a primary cancer in another part of the body. Secondary tumors are about three times more common than primary tumors of the brain. Solitary metastasized brain cancers may occur but are less common than multiple tumors. Most often, cancers that spread to the brain to cause secondary brain tumors originate in the lung, breast, kidney, or from melanomas in the skin.
All metastatic brain tumors are malignant. This report discusses primary malignant brain tumors.
Primary Glioma Brain Tumors
About 80% of malignant primary brain tumors are known collectively as gliomas. Gliomas are not a specific type of cancer but are a term used to describe tumors that originate in glial cells. Glial cells are the building-block cells of the connective, or supportive, tissue in the central nervous system.
Gliomas are classified into four grades that reflect the degree of malignancy. Grades I and II are considered low-grade and grades III and IV are considered high-grade. Grades I and II are the slowest-growing and least malignant; grade I tumors are generally considered borderline between benign and malignant. Grade III tumors are considered malignant and grow at a moderate rate. Grade IV tumors, such as glioblastoma multiforme, are the fastest-growing and most malignant primary brain tumors.
Gliomas may develop from several types of glial cells.Astrocytomas are primary brain tumors derived from astrocytes, which are star-shaped glial cells. Astrocytomas account for about 60% of all malignant primary brain tumors. Astrocytoma tumor types by grade include:
- Grade I. Pilocytic astrocytoma is one of the most common types of glioma in children
- Grade II. Diffuse astrocytoma (also called low-grade astrocytoma) typically occurs in men and women ages 20 - 60
- Grade III. Anaplastic astrocytoma typically occurs in adults ages 30 - 60 and is more common among men than women.
- Grade IV. Glioblastoma multiforme (GBM), also called glioblastoma, accounts for about 50% of all astrocytomas and is one of the deadliest types of brain tumors. These highly malignant aggressive tumors grow rapidly. They are most common in older adults (ages 50 - 70), particularly men. Only about 10% of childhood brain tumors are glioblastomas.
Oligodendrogliomas develop from oligodendrocyte glial cells, which form the protective coatings around nerve cells. Oligodendrogliomas are classified as either low-grade (grade II) or anaplastic (grade III). Pure oligodendrogliomas, however, are rare. In most cases they occur in mixed gliomas. Oligodendrogliomas usually occur in younger and middle-aged adults.
Ependymomas are derived from ependymal cells, which line the ventricles (fluid-filled cavities) in the lower part of the brain and the central canal of the spinal cord. They are one of the most common types of brain tumor in children. They can also occur in adults in their 40s and 50s. Ependymomas are divided into four categories: Myxopapillar ependymomas (grade I), subependymomas (grade I), ependymomas (grade II), and anaplastic aependymomas (grades III and IV).
Mixed gliomas contain a mixture of malignant gliomas. About half of these tumors contain cancerous oligodendrocytes and astrocytes.
Gliomas may also contain cancer cells derived from brain cells other than glial cells.
Location of Gliomas. Gliomas are also described by the location of the tumor. Examples include:
- Brain stem gliomas develop in the lowest portion of the brain. The brain stem connects the cerebrum (the largest part of the brain) to the spinal cord. Between 10 - 20% of brain tumors in children are brain stem gliomas. Most of these tumors are astrocytomas.
- Cerebellar astrocytomas occur in the cerebellum part of the brain, which controls balance and coordination.
- Optic gliomas occur in the optic nerve and other parts of the eye. They primarily affect children younger than age 10. About 20% of children with the genetic disorder neurofibromatosis 1 (NF-1) develop optic gliomas. Pilocytic astrocytoma and fibrillary astrocytoma are common types of optic gliomas.
Primary Non-Glioma Brain Tumors
Malignant types of non-glioma brain tumors include:
- Medulloblastomas. Medulloblastomas are always located in the cerebellum, which is at the base and toward the back of the brain. These fast-growing high-grade tumors represent about 15 - 20% of pediatric brain tumors and 20% of adult brain tumors.
- Pituitary Adenomas. Pituitary tumors (also called pituitary adenomas) comprise about 10% of primary brain tumors and are often benign, slow-growing masses in the pituitary gland. They are more common in women than men.
- Central Nervous System Lymphomas. Central nervous system (CNS) lymphomas can affect both people with healthy immune systems and those who are immunocompromised due to other medical conditions (recipients of organ transplants, patients infected with HIV). CNS lymphomas occur most often in the cerebral hemisphere but can also develop in cerebrospinal fluid, eyes, and spinal cord. [For more information, see In-Depth Report #84: Non-Hodgkin's lymphoma.]
Benign types of non-glioma brain tumors include:
- Meningiomas. Meningiomas are usually benign tumors that develop in the membranes that cover the brain and spinal cord (the meninges).
- Meningiomas account for about 25% of all primary brain tumors and are most common in women in their 60s and 70s. Meningiomas are classified as benign meningioma (grade I), atypical meningioma (grade II), and anaplastic meningioma (grade III).
Causes
Genetics
Only 5 - 10% of primary brain tumors are associated with genetic disorders. These inherited conditions and associated genes include:
- Von Recklinghausen disease, also called neurofibromatosis 1 (NF1 gene) and neurofibromatosis 2 (NF2 gene)
- Turcot syndrome (APC gene)
- Gorlin syndrome, also called basal cell nevus syndrome (PTCH gene)
- Tuberous sclerosis (TSC1 and TSC2 genes)
- Li-Fraumeni syndrome (TP53 gene)
Certain types of brain tumors are specifically linked with these genetic conditions. For example, neurofibromatosis 1 is associated with about 15% of cases of pilocytic astrocytomas, the most common type of childhood glioma.
Many different cancer-causing genes (oncogenes) are involved in cancer. Growth factors are a particularly important type of oncogene associated with brain tumors. Growth factors attach to receptors (connectors) that stimulate cell growth. Epidermal growth factor receptor (EGFR) has been shown to play a role in high-grade brain tumors such as glioblastoma multiforme. Knowing the molecular origin of a brain tumor may help determine the treatment course, both for standard chemotherapy and "targeted therapy" biologic drugs.
Most genetic abnormalities that cause brain tumors are not inherited but occur as a result of environmental or other factors that affect genetic materials (DNA) in the cells. Researchers are studying various environmental factors (such as viruses, hormones, chemicals, and radiation) that may trigger the genetic disruptions that lead to brain tumors in susceptible individuals. They are also working to identify the specific genes that are affected by these environmental triggers.
Risk Factors
Primary malignant brain tumors account for about 2% of all cancers. However, brain and spinal cord tumors are the second most common type of cancer in children (after leukemia). According to the American Cancer Society, about 23,000 people in the United States are diagnosed each year with a malignant brain or spinal cord tumor.
Gender
In general, brain tumors are slightly more likely to occur in men than in women. Some specific types of brain tumors, such as meningiomas, are more common in women.
Age
Most brain tumors in adults occur between the ages of 65 - 79. Brain tumors also tend to occur in children younger than age 8. In children, malignant brain tumors are the leading cause of death from solid tumors (non-blood cell cancers).
Ethnicity
The risk for primary brain tumors in Caucasians is higher, as much as two-fold with gliomas, than in people of other races.
Environmental or Occupational Risk Factors
Exposure to ionizing radiation, usually from radiation therapy, is the only environmental risk factor that has been definitively linked to brain tumors. People who receive radiation therapy to the head during cancer treatment have an increased risk of developing brain tumors 10 - 15 years later. Workers in the nuclear industry are also at increased risk.
Despite much research, there is no evidence that electromagnetic field exposure from power lines or household appliances poses any risk. Several recent epidemiological studies have examined the cancer risk of wireless devices such as cellular (mobile) phones, which emit radiofrequency (RF) energy. To date, the studies do not show an association between cell phone use and common brain tumors like gliomas and meningiomas. However, more research is needed to examine potential long-term effects, particularly for children and adolescents.
Researchers have also investigated a number of metals and chemicals including vinyl chloride, petroleum products, lead, arsenic, mercury, and pesticides. To date, there has been no clear evidence that implicates any specific industrial chemical or metal. Research continues.
Medical Conditions
People with impaired immune systems have an increased risk of developing central nervous system lymphomas. Organ transplantation, HIV infection, and chemotherapy are some medical factors that can weaken the immune system.
Prognosis
Recent advances in surgical and radiation treatments have significantly extended average survival times of patients with brain tumors. These new treatments can often help reduce the size and progression of malignant gliomas.
Survival Rates
The survival rates in people with brain tumors depend on many different variables:
- Type of tumor (such as astrocytoma, oligodendroglioma, or ependymoma)
- Location and size of tumor (these factors affect whether or not the tumor can be surgically removed)
- Tumor grade
- Patient's age
- Patient's ability to function
- How far the tumor has spread
Patients with some types of tumors have relatively good survival rates. Five-year survival rates for patients with ependymoma and oligodendroglioma are, respectively, 86% and 82% for people ages 20 - 44, and 69% and 48% for patients ages 55 - 64.
Glioblastoma multiforme has the worst prognosis with 5-year survival rates of only 14% for people ages 20 - 44, and 1% for patients age 55 - 64. Survival rates tend to be highest for younger patients and decrease with age.
Grading Tumors
Malignant primary brain tumors are classified according to tumor grade. Grade I is the least cancerous, and Grades III and IV are the most dangerous. Grading a tumor can help predict its growth rate and tendency to spread. Grading is based on the appearance of the tumor cells as seen under a microscope.
- Lower-grade (I and II) tumor cells are well defined and almost normal-looking. Some primary low-grade brain tumors are curable by surgery alone, and some are curable by surgery and radiotherapy. Low-grade tumors tend to have the best favorable survival rates and high-grade the poorest outlook. However, this is not always the case. For example, some low-grade II gliomas are at very high risk for progression.
- Higher-grade (III and IV) tumor cells are abnormal appearing and are more diffuse, which indicates more aggressive behavior. (High-grade brain tumors usually require surgery, radiotherapy, chemotherapy, and possibly investigational treatments.)
In tumors that contain a mixture of different-grade cells, the tumor is graded according to the highest-grade cells in the mixture, even if there are very few of them.
Specific Effects of Tumors on Function
Brain tumors can cause seizures, mental changes, and mood, personality, and emotional changes. Tumors may also impair muscle function, hearing, vision, speech, and other neurologic activities. Such effects can be very difficult for both patients and caregivers. Numerous treatments are available that can help alleviate these complications. Patients and family members should discuss these options with their doctors.
Effects in Children. Advancements in treatment have dramatically increased survival rates for children with brain tumors. About 75% of children survive at least 5 years after being diagnosed with a brain tumor. Unfortunately, many childhood brain tumor survivors are at risk for long-term neurological complications.
Children younger than age 7 (and particularly those younger than age 3 years) appear to have the greatest risk for cognitive problems. These problems may result from the tumor and from treatment (cranial radiation therapy, chemotherapy that penetrates the blood-brain barrier). Long-term cognitive problems include difficulties with attention and concentration, memory, mental processing of information, visual perception skills, and problems with planning, insight, initiative, and organizational competencies. Parents need to make sure that children receive appropriate supportive services and educational accommodation at their schools.
Symptoms
Brain tumors produce a variety of symptoms, ranging from headache to seizure. They are great mimics of other neurologic disorders. Symptoms occur if the tumor directly damages the nerves in the brain or central nervous system, or if its growth puts pressure on the brain. Symptoms may be subtle and gradually become worse, or they may occur very rapidly.
Headache
Headache is probably the most frequent symptom of a brain tumor. However, headaches are very common, and the great majority of headaches are not caused by brain tumors. [For information on some of the many causes of headaches, see In-Depth Reports #97: Migraine headache; #11: Tension headache; and #99: Cluster headache.]
Headaches caused by brain tumors vary depending on the tumor's location and other factors. Symptoms that may be associated with brain tumors include:
- Steady and worse headache that occurs upon waking in the morning, then clears up within a few hours
- Persistent non-migraine headache that occurs while sleeping and is also accompanied by at least one other symptom (such as vomiting or confusion)
- Headache that may or may not be throbbing
- Headache accompanied by double vision, weakness, or numbness
- Headache that may worsen with coughing or exercise or with a change in body position
Gastrointestinal Symptoms
Gastrointestinal symptoms, including nausea and vomiting, are also common.
Seizures
About half of patients with brain tumors have a seizure. It is a common symptom of brain tumors in older adults. (However, very few first seizures are caused by a brain tumor.)
- Tumors are more likely to be localized and affect one area of the brain. If so, they can cause partial seizures. In this case, a person does not lose consciousness but may have confusion, jerking movements, tingling, or odd mental and emotional events.
- Generalized seizures, which can cause loss of consciousness, are less common, since they are caused by disturbances of nerve cells in diffuse areas of the brain.
Mental Changes
Sometimes the only symptoms of brain tumors are mental changes, which may include:
- Memory loss
- Impaired concentration
- Problems with reasoning
- Changes in personality and behavior
- Increased sleep
Other Significant Symptoms
- Gradual loss of movement or sensation in an arm or leg
- Unsteadiness and problems with balance
- Unexpected visual disturbance (especially if it is associated with headache), including vision loss (usually of peripheral vision) in one or both eyes or double vision
- Hearing loss with or without dizziness
- Speech difficulty
Diagnosis
A neurological exam is usually the first test given when a patient complains of symptoms that suggest a brain tumor. The exam includes checking eye movements, hearing, sensation, muscle strength, sense of smell, and balance and coordination. The doctor will also test mental state and memory.
Imaging Techniques
Advanced imaging techniques have dramatically improved the diagnosis of brain tumors.
Magnetic Resonance Imaging. Magnetic resonance imaging (MRI) is the standard crucial step for diagnosing a brain tumor. It provides pictures from various angles that can help doctors to construct a three-dimensional image of the tumor. It gives a clear picture of tumors near bones, smaller tumors, brain stem tumors, and low-grade tumors. MRI is also useful during surgery to show tumor bulk, for accurately mapping the brain, and for detecting response to therapy.
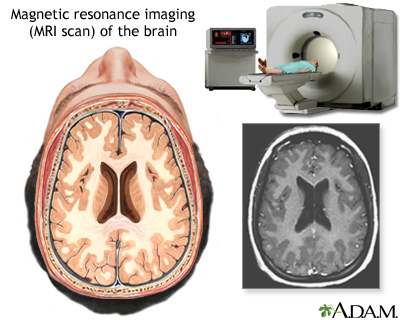
Computed Tomography. Computed tomography (CT) uses a sophisticated x-ray machine and a computer to create a detailed picture of the body's tissues and structures. It is not as sensitive as an MRI in detecting small tumors, brain stem tumors, and low-grade tumors. It is useful in certain situations, however. Often, doctors will inject the patient with a contrast material to make it easier to see abnormal tissues. A CT scan helps locate the tumor and can sometimes help determine its type. It can also help detect swelling, bleeding, and associated conditions. In addition, computed tomography is used to evaluate the effectiveness of treatments and watch for tumor recurrence.
Positron Emission Tomography. Positron emission tomography (PET) provides a picture of the brain's activity rather than its structure by tracking a sugar that has been labeled with a radioactive tracer. It is sometimes able to distinguish between recurrent tumor cells and dead cells or scar tissue caused by radiation therapy. PET is not routinely used for diagnosis, but it may supplement MRIs to help determine tumor grade after a diagnosis. Data from PET may also help improve the accuracy of newer radiosurgery techniques. PET scans are often done along with a CT scan.
Other Imaging Techniques. Numerous other advanced or investigational imaging techniques include:
- Single photon emission tomography (SPECT) is similar to PET but is not as effective in distinguishing tumor cells from destroyed tissue after treatments. It may be used after CT or MRI to help distinguish between low-grade and high-grade tumors.
- Magnetoencephalography (MEG) scans measure the magnetic fields created by nerve cells as they produce electrical currents. It is used to evaluate functioning in various parts of the brain. However, this procedure is not widely available.
- MRI angiography evaluates blood flow. MRI angiography is usually limited to planning surgical removal of a tumor suspected of having a large blood supply.
Lumbar Puncture (Spinal Tap)
A spinal tap (lumbar puncture) is used to obtain a sample of cerebrospinal fluid, which is examined for the presence of tumor cells. Spinal fluid may also be examined for the presence of certain tumor markers (substances that indicate the presence of a tumor). However, most primary brain tumors do not have identified tumor markers.
A computed tomography (CT) scan or magnetic resonance imaging (MRI) should generally be performed before a lumbar procedure to make sure that the procedure can be performed safely.
Biopsy
A biopsy is a surgical procedure in which a small sample of tissue is taken from the suspected tumor and examined under a microscope for malignancy. The results of the biopsy also provide information on the cancer cell type. Biopsies may be performed as part of surgery to remove a tumor, or as a separate diagnostic procedure.
In some cases, such as with brain stem gliomas, a standard biopsy might be too hazardous because removing any healthy tissue from this area can affect vital functions. In these cases, surgeons can use alternative techniques such as needle biopsy and stereotaxic biopsy. Stereotaxic biopsy is a computer-directed type of needle biopsy that uses images provided from MRI or CT scans to provide precise information on the tumor's location.
Treatment
Standard Treatments
The standard approach for treating brain tumors is to reduce the tumor as much as possible using surgery, radiation therapy (also called radiotherapy), or chemotherapy. Such treatments are used alone or, more commonly, in combination with one another.
The intensity, combination, and sequence of treatments depend on the brain tumor type (there are over 100 types of brain tumors), its size and location, and patient age, health status, and medical history. Unlike other types of cancer, there is no formal staging system for brain tumors.
With some very slow-growing cancers, such as those that occur in the midbrain or optic nerve pathway, patients may be closely observed and not treated until the tumor shows signs of growth.
Other Treatments
TTF Therapy. Tumor Treating Fields (TTF) therapy uses a low-intensity electrical field to disrupt the rapid division of cancer cells. In 2011, the FDA approved the NovoTTF-100A System to treat adults with glioblastoma multiforme that has recurred or progressed despite chemotherapy and radiation. This new device uses electrodes placed on the patient’s scalp to deliver alternating electrical fields to the tumor site. The device is portable and can be used at home
Investigational Treatments. Patients can enroll in clinical trials that are researching new treatments for brain tumors.
Emotional Support for Patients and Caregivers
Support for patients and their families is a critical component of treatment and management. Helpful measures include:
- Any physical impairment that could benefit from home equipment or physical therapy should be identified and treated.
- Patients should discuss emotional as well as physical issues with their doctors. Depression, for instance, can be medically treated. Caregivers should also seek help for the inevitable stress, depression, and tension arising from their difficult role.
- Relaxation techniques, meditation, and spiritual resources may be helpful. Support groups are beneficial, but mental health professionals recommend separate groups for patients and their families.
Surgery
Surgery is usually the first step in treating most brain tumors. In some cases, however, such as most brain stem gliomas and other tumors located deep inside the brain, it may be too dangerous to perform surgery. The objective of most brain tumor surgeries is to remove or reduce as much of the tumor's bulk as possible. By reducing the tumor's size, other therapies, particularly radiotherapy, can be more effective.
Craniotomy
The standard surgical procedure is called craniotomy.
- The neurosurgeon removes a piece of skull bone to expose the area of brain over the tumor.
- The tumor is located and then removed.
There are various surgical options for breaking down and removing the tumor. They include:
- Standard surgical procedures
- Laser microsurgery (which produces focused heat that vaporizes tumor cells)
- Ultrasonic aspiration (which uses ultrasound to break the glioma tumor into small pieces, which are then suctioned out)
Relatively benign, grade I gliomas may be treated only by surgery. Most malignant tumors require additional treatments, including repeat surgery.
Imaging techniques, such as CT and MRI, are used along with surgery to help map the area of the tumor in the brain.
The neurosurgeon's skill in removing the tumor as completely as possible is critical to survival. No one should be shy about asking the surgeon the number of similar procedures they have performed. (Asking for complication rates may not be useful, since a very experienced surgeon might operate on many high-risk patients.)
Shunt Placement
Sometimes a brain tumor can create blockage and cerebrospinal fluid accumulates excessively in the skull, causing increased intracranial pressure. In these cases, a surgeon may implant a ventriculoperitoneal shunt (VP) to help drain the fluid. The procedure involves placing a thin catheter into a brain ventricle and connecting it to a catheter that is tunneled into the abdominal (peritoneal) cavity. A pump that controls the flow of fluid is attached to both catheters.
Risks and Complications of Surgery
The most serious concern of brain surgery is preserving brain function. Surgeons will try to be conservative in their approach so as to limit removing tissue that may cause a loss of function. Bleeding and blood clots are other complications. Postsurgical complications include swelling in the brain, which is typically treated with corticosteroid drugs. Steps are taken to reduce the risk of blood clots during the postoperative period.
Radiation
Radiation therapy, also called radiotherapy, plays a central role in the treatment of most brain tumors.
Various radiation treatments are used. Radiation is usually given externally, from a source outside the body that directs radiation beams. In some cases, internal radiation may be used as a booster to external-beam radiation. Internal radiation (also called interstitial radiation) generally involves brachytherapy, which uses radioactive "seeds" implanted directly in the tumor site.
Radiotherapy after Surgery. Even when it appears that the entire tumor has been surgically removed, microscopic cancer cells often remain in the surrounding brain tissue. Radiation targets the residual tumor with the goal of reducing its size or stopping its progression. If the entire tumor cannot be removed safely, postoperative radiotherapy is often recommended. Even some benign gliomas may need radiation, since they may become life threatening if their growth is not controlled.
Radiotherapy When Surgery Is Not Appropriate. Radiotherapy may be used instead of surgery for inaccessible tumors or for tumors that have properties that are particularly responsive to radiotherapy.
Radiotherapy and Chemotherapy (Radiochemotherapy). Combining chemotherapy with radiotherapy is beneficial for some patients with high-grade tumors.
Conventional Radiotherapy
Conventional radiotherapy uses external beams aimed directly at the tumor and is usually recommended for large or infiltrating tumors. It begins about a week after surgery and continues on an outpatient basis 5 days per week for 6 weeks. Older adults tend to have a more limited response to external-beam radiation therapy than younger people. Conventional external-beam radiation techniques include:
- Three-dimensional conformal radiation therapy (3D-CRT) uses computer-generated imaging scans to map the tumor’s location. Radiation beams are then used that conform to the three-dimensional shape of the tumor.
- Intensity-modulated radiation therapy (IMRT) is a more advanced and higher-dose form of 3D-CRT.
- Conformalproton beam radiation therapy is also similar to 3D-CRT but uses proton beams instead of x-ray energy. It is not yet widely available.
Stereotactic Radiosurgery
Stereotactic radiosurgery, also called stereotactic radiotherapy or stereotaxy, is an alternative to conventional radiotherapy that allows highly targeted radiation to be delivered directly to small tumors while avoiding healthy brain tissue. The term radiosurgery is used because the destruction is so precise that it acts almost like a surgical knife. Benefits of stereotactic radiosurgery include:
- Stereotaxy allows precisely focused, high-dose beams to be delivered to gliomas smaller than 1.25 inches in diameter with less damage to surrounding tissues.
- Stereotactic radiosurgery can help reach small tumors located deep in the brain that were previously considered inoperable.
- Sometimes with stereotaxy only a single treatment may be needed.
- Unlike traditional radiotherapy, stereotactic radiotherapy can be repeated, so it is useful for recurrent tumors when a patient has already received standard radiation treatments.
- Combining stereotaxy with techniques that evaluate speech and other mental functions in patients who are awake during the procedure can allow removal of brain tissue with a lower risk for complications in areas that affect such functioning.
The Planning Procedure. Stereotactic radiosurgery usually begins with a series of steps designed to plan the radiation target:
- First, the patient is given a local anesthetic. In the standard operation, the patient's head must be totally immobilized by screwing a device known as a stereotactic frame into the patient's skull. (The frame procedure is effective only on brain tumors that have regular margins.) The frame is removed as soon as the whole procedure has been completed (about 3 - 4 hours).
- A three-dimensional map, usually using magnetic resonance imaging (MRI) scans, is made of the patient's brain.
- A computer program calculates dosage levels and specific areas for radiation targeting.
Advanced imaging techniques are now allowing frameless stereotaxy, which eliminates the frame and may be effective on more tumors.
Delivery of Radiation Beams. Once the preliminary planning stage has been completed, treatment begins. Several advanced machines, such as the gamma knife and adapted linear accelerator (LINAC), are used with stereotaxy to deliver very focused beams of radiation. Actual treatment takes 10 minutes to 1 hour.
- The gamma knife uses gamma rays that are sent from multiple points to converge at a single point on the tumor. Although each gamma-ray beam is very low dosage, when the beams converge, the intensity and destructive power is very high. The gamma knife is used only on very small tumors and so is generally useful as a booster after standard radiation, surgery, chemotherapy, or combinations.
- The linear accelerator (LINAC) produces protons (positively-charged atomic particles) in patterns that are matched to the tumor shape. The patient is positioned on a bed that can be moved to allow flexible positioning. It allows treatment over multiple sessions of small doses (fractionated stereotactic radiotherapy), instead of a single session. This means that larger tumors can be treated.
Drugs Used With Radiation
Researchers are studying drugs that may be used along with radiation to increase the effectiveness of the treatment.
Radioprotectors. Drugs such as amifosistine (Ethyol, generic) may protect healthy cells during radiation.
Radiosensitizers. Drugs such as fluorouracil (5-FU) and cisplatin (Platinol, generic) may help make cancerous cells more sensitive to radiation.
Side Effects of Radiation
Common Side Effects. Side effects of radiotherapy vary depending on the tumor type and radiation treatment. Side effects may include hair loss, fatigue, and nausea and vomiting. Skin irritation and sensitivity may develop in the areas being treated. To prevent further irritation, avoid scratching or rubbing, avoid direct sunlight and heating pads, and do not attempt to treat the symptoms yourself. (Ask your doctor or radiation therapist for advice.) Brain swelling (edema) is another common radiotherapy side effect, which can sometimes cause an increase in neurologic symptoms. Edema can be treated with steroids.
Tissue Injury. Radiation necrosis (total destruction of nearby healthy tissue) occurs in about 25% of patients treated with intensive radiation. Radiation necrosis can cause brain swelling and reduction in mental functions. The condition is treated with steroids. If steroids prove ineffective, surgery may be required to remove the damaged tissue.
New Tumors. Radiation therapy for childhood cancer is the most important risk factor for developing new brain and spinal column tumors. The risk appears greatest for children who received radiation therapy before age 5. It appears that the risk of second primary tumors increases in relation to the radiation dose used to treat the first cancer.
Stroke. Survivors of childhood brain tumors who were treated with high doses of cranial radiation (especially doses greater than 50Gy) may be at increased risk of having a stroke later in life. In a study of nearly 2,000 brain tumor survivors, the average length of time from cancer diagnosis to stroke was 14 years.
Chemotherapy
Chemotherapy uses drugs to kill or alter cancer cells. Chemotherapy is not an effective initial treatment for low-grade brain tumors, mostly because standard drugs have a hard time passing into the brain because of how the brain protects itself (the blood-brain barrier). In addition, not all types of brain tumors respond to chemotherapy. In general, chemotherapy for brain tumors is usually administered following surgery or radiation therapy.
The type of drug determines how it is administered. "Systemic delivery" drugs, which pass to the brain from the bloodstream, may be given by mouth, injected into a vein through an IV, or injected into an artery or a muscle. "Local delivery" drugs are placed within or around the brain tumor.
Newer delivery methods to overcome some of these problems include:
- Interstitial chemotherapy uses disc-shaped polymer wafers (known as Gliadel wafers) soaked with carmustine, the standard chemotherapeutic drug for brain cancer. The surgeon implants the wafer directly into the surgical cavity after a tumor is removed.
- Intrathecal chemotherapy delivers chemotherapeutic drugs directly into the spinal fluid.
- Intra-arterial chemotherapy delivers high-dose chemotherapy into arteries in the brain using tiny catheters.
- Convection-enhanced delivery (CED) involves placing catheters into the brain tumor or nearby brain tissue to deliver slowly and continuously a cancer drug over several days.
Chemotherapy Drugs and Regimens
Many different drugs, and drug combinations, are used for chemotherapy. Standard ones include:
Temozolomide (Temodar, generic). Temozolomide is approved for adult patients with anaplastic astrocytoma that did not respond to other treatments. It is also approved for use during and after radiation therapy for patients newly diagnosed with glioblastoma multiforme. The current first-line treatment for patients with glioblastoma is combined radiotherapy and temozolomide, followed by monthly doses of temozolomide after radiation treatment ends. The drug may work best for patients with a specific genotype. Temozolomide's side effects are relatively minor, but may include constipation, nausea and vomiting, fatigue, and headache. The drug is taken by mouth as a pill.
Carmustine (BCNU, BiCNU). Carmustine is used to treat many types of brain tumors, including glioblastoma, medulloblastoma, and astrocytoma. Carmustine is usually administered into the vein by IV. It can also be delivered through a wafer implant (Gliadel), which is surgically placed into the brain cavity after tumor removal. If carmustine is administered intravenously, side effects may include nausea and vomiting, fatigue, respiratory problems, and lung scarring (pulmonary fibrosis). Intravenous carmustine may cause bone marrow impairment, which results in decreased production of blood cells (a condition called myelosuppression). If carmustine is delivered through a wafer, side effects may include seizures, brain swelling, and infection within the brain cavity.
PCV Drug Regimen. PCV is an abbreviation for a chemotherapy regimen that combines procarbazine (Matulane), lomustine (Ceenu, CCNU), and vincristine (Oncovin, generic). PCV is commonly used to treat oligodendrogliomas and mixed oligoastrocytomas. The drugs may also be used alone or in other combinations. Procarbazine and lomustine are taken by mouth. Vincristine is given by either injection or IV. These drugs can cause significant side effects, including a drop in blood cell counts, nausea and vomiting, constipation, fatigue, and mouth sores. Procarbazine can cause high blood pressure when taken with foods high in tyramine. Patients should avoid foods such as beer, red wine, cheese, chocolate, processed meat, yogurt, and certain fruits and vegetables.
Platinum-Based Drugs. Cisplatin (Platinol, generic) and carboplatin (Paraplatin, generic) are standard cancer drugs that are sometimes used to treat glioma, medulloblastoma, and other types of brain tumors. These drugs are delivered by IV. In addition to nausea and vomiting, carboplatin can cause hair loss, and cisplatin can cause muscle weakness.
Other Chemotherapy Drugs. Researchers are investigating whether drugs used to treat other types of cancer may have benefits for brain tumors. These drugs include:
- Tamoxifen (Nolvadex, generic) and paclitaxel (Taxol, generic), which are used to treat breast cancer
- Topotecan (Hycamtin, generic), which is used to treat ovarian and lung cancers
- Vorinostat (Zolinza), which is approved for treatment of cutaneous T-cell lymphoma
Irinotecan (Camptostar, generic) is another cancer drug that is being studied in combination treatment.
Biologic Drugs (Targeted Therapy)
Traditional chemotherapy drugs can be effective, but because they do not distinguish between healthy and cancerous cells their generalized toxicity can cause severe side effects. Targeted biologic therapies work on a molecular level by blocking specific mechanisms associated with cancer cell growth and division. Because they selectively target cancerous cells, these biologic drugs may induce less severe side effects. In addition, these drugs hold the promise of creating options for more individualized cancer treatment based on a patient's genotype.
Bevacizumab (Avastin). Bevacizumab (Avastin) is a biologic drug that blocks the growth of blood vessels that feed tumors (a process called angiogenesis). It is approved for the treatment of glioblastoma in patients whose brain cancer has continued to progress after prior treatment with chemotherapy and radiation. Bevacizumab was the first targeted therapy approved for brain tumors, and the first new treatment for glioblastoma in more than a decade.
Investigational Targeted Therapies. Targeted therapies being tested in clinical trials include:
- Vaccines are among the most promising immunotherapies being tested for slowing the progression of glioblastoma multiforme. Unlike conventional vaccines that are used to prevent disease, these vaccines are used as a cancer treatment. CDX-110 (Rindopepimut) and DCVax-Brain are two such vaccines.
- Tyrosine kinase inhibitor drugs block proteins involved in tumor cell growth and production. Tyrosine kinase inhibitors that target EGFR [such as erlotinib (Tarceva), imatinib (Gleevec), and gefitinib (Iressa)] are being studied for their effects on brain tumors.
- MTOR inhibitors target other enzymes involved in cell growth and replication. These drugs are commonly used to suppress the immune system to prevent rejection after organ transplantation. Everolimus (Afinitor, Afinitor Disperz) is approved for treatment of benign brain tumors associated with a rare genetic condition and for treatment of a rare pediatric brain tumor called subependymal giant cell astrocytoma (SEGA). Everolimus is related to sirolimus (Rapamune) and tacrolimus (Prograf, generic), which are also being investigated for brain tumor treatment.
Treatment of Complications
Peritumoral Edema
Some tumors, particularly medulloblastomas, interfere with the flow of cerebrospinal fluid and cause hydrocephalus (accumulation of fluid in the skull), which in turn causes a build-up fluid in the ventricles (the cavities) in the brain. Symptoms of peritumoral edema include nausea and vomiting, severe headaches, lethargy, difficulty staying awake, seizures, visual impairment, irritability, and tiredness.
Corticosteroids (commonly called steroids), such as dexamethasone (Decadron), are used to treat peritumoral edema. Side effects include high blood pressure, mood swings, increased risk of infection, stronger appetite, facial swelling, and fluid retention.
A shunt procedure may be performed to drain fluid. Shunts are flexible tubes used to reroute and drain the fluid.
Seizures
Seizures are common in brain tumor cases, with younger patients having higher risks than older ones. Anti-seizure (anti-convulsant) medications, such as carbamazepine or phenobarbital, may treat seizures and are helpful in preventing recurrence. These drugs are not useful in preventing a first seizure, however, and they should not be used routinely to treat patients with newly diagnosed brain tumors. Anti-seizure medications should be used only for patients who have experienced a seizure.
Anti-seizure medications can interact with some of the chemotherapies used to treat brain cancers, including paclitaxel, irinotecan, interferon, and retinoic acid. Patients should discuss these interactions with their doctors.
Depression
Antidepressants can help treat the emotional side effects of associated with brain tumors. Support groups can also have great benefit for both patients and families.
Resources
- www.abta.org -- American Brain Tumor Association
- www.cbtf.org -- Children's Brain Tumor Foundation
- www.virtualtrials.com -- Musella Foundation for Brain Tumor Research and Information
- www.braintumor.org -- National Brain Tumor Society
- www.neurosurgery.org -- American Association of Neurologic Surgeons
- www.cancer.org -- American Cancer Society
- www.cancer.gov -- National Cancer Institute
- www.asco.org -- American Society for Clinical Oncology
- www.cancer.gov/clinicaltrials -- Find clinical trials
- www.radiologyinfo.org -- RadiologyInfo
- www.cancer.net -- Cancer.Net
References
Bowers DC, Liu Y, Leisenring W, McNeil E, Stovall M, Gurney JG, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006 Nov 20;24(33):5277-82. Epub 2006 Nov 6.
Buckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007 Oct;82(10):1271-86.
Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010 Mar;67(3):279-83.
Frei P, Poulsen AH, Johansen C, Olsen JH, Steding-Jessen M, Schüz J. Use of mobile phones and risk of brain tumours: update of Danish cohort study. BMJ. 2011 Oct 19;343:d6387. doi: 10.1136/bmj.d6387.
Gerstner ER, Batchelor TT. Primary central nervous system lymphoma. Arch Neurol. 2010 Mar;67(3):291-7.
INTERPHONE Study Group. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 2010 Jun;39(3):675-94. Epub 2010 May 17.
Iwamoto FM, Fine HA. Bevacizumab for malignant gliomas. Arch Neurol. 2010 Mar;67(3):285-8.
Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45-68. Epub 2009 Dec. 4.
Mitchell DA, Sampson JH. Toward effective immunotherapy for the treatment of malignant brain tumors. Neurotherapeutics. 2009 Jul;6(3):527-38.
Nathan PC, Patel SK, Dilley K, Goldsby R, Harvey J, Jacobsen C, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children's Oncology Group. Arch Pediatr Adolesc Med. 2007 Aug;161(8):798-806.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Central nervous system cancers. V.2.2012.
Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ, Hammond S, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006 Nov 1;98(21):1528-37.
Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012 May 26;379(9830):1984-96. Epub 2012 Apr 16.
Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN. Molecularly targeted therapy for malignant glioma. Cancer. 2007 Jul 1;110(1):13-24.
Stupp R, Roila F; ESMO Guidelines Working Group. Malignant glioma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009 May;20 Suppl 4:126-8.
Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010;362:1119-1127.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008 Jul 31;359(5):492-507.
Wilne S, Koller K, Collier J, Kennedy C, Grundy R, Walker D. The diagnosis of brain tumours in children: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumour. Arch Dis Child. 2010 Jul;95(7):534-9. Epub 2010 Apr 6.
|
Review Date:
12/18/2012 Reviewed By: Harvey Simon, MD, Editor-in-Chief, Associate Professor of Medicine, Harvard Medical School; Physician, Massachusetts General Hospital. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M., Inc. |